Increased gut permeability and intestinal inflammation precede arthritis onset in the adjuvant-induced model of arthritis
ABSTRACT
BACKGROUND
Intestinal inflammation, dysbiosis, intestinal permeability (IP), and bacterial translocation (BT) have been identified in patients with spondyloarthritis but the time at which they appear and their contribution to the pathogenesis of the disease is still a matter of debate.
OBJECTIVES
To study the time-course of intestinal inflammation (I-Inf), IP, microbiota modification BT in a rat model of reactive arthritis, the adjuvant-induced arthritis model (AIA).
METHODS
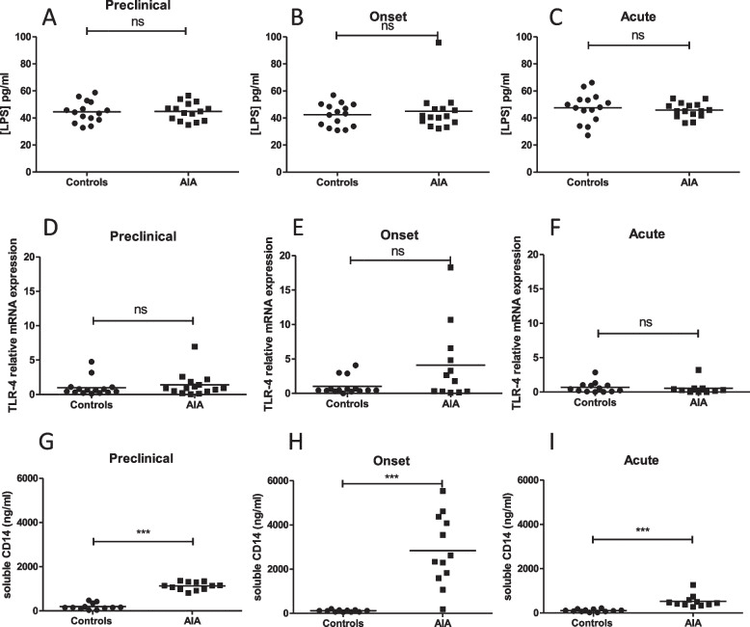
Analysis was performed at 3 phases of arthritis in control and AIA rats: preclinical phase (day 4), onset phase (day 11), and acute phase (day 28). IP was assessed by measuring levels of zonulin and ileal mRNA expression of zonulin. I-inf was assessed by lymphocyte count from rat ileum and by measuring ileal mRNA expression of proinflammatory cytokines. The integrity of the intestinal barrier was evaluated by levels of iFABP. BT and gut microbiota were assessed by LPS, soluble CD14 levels, and 16S RNA sequencing in mesenteric lymph node and by 16S rRNA sequencing in stool, respectively.
RESULTS
Plasma zonulin levels increased at the preclinical and onset phase in the AIA group. Plasma levels of iFABP were increased in AIA rats at all stages of the arthritis course. The preclinical phase was characterized by a transient dysbiosis and increased mRNA ileal expression of IL-8, IL-33, and IL-17. At the onset phase, TNF-α, IL-23p19, and IL-8 mRNA expression were increased. No changes in cytokines mRNA expression were observed at the acute phase. Increased CD4^+^ and CD8^+^ T cell number was measured in the AIA ileum at day 4 and day 11. No increase in BT was observed.
CONCLUSION
These data show that intestinal changes precede the development of arthritis but argue against a strict "correlative" model in which arthritis and gut changes are inseparable.